Tech Talk: pH Electrode Operation & Replacement
pH Electrode Basics 101
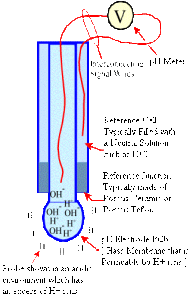
Design & Construction – pH electrodes are constructed from a special composition glass which senses the hydrogen ion concentration. This glass is typically composed of alkali metal ions. The alkali metal ions in the glass and the hydrogen ions in solution undergo an ion exchange reaction, generating a potential difference. In a combination pH electrode, the most widely used variety, there are actually two electrodes in one body.
One portion is called the measuring electrode, the other the reference electrode. The potential generated at the junction site of the measuring electrode is due to the free hydrogen ions present in the solution.
Storage & Handling – Due to their design, pH electrodes must be kept moist at all times. In other words, for the electrode to operate properly, glass needs to be hydrated as hydration is required for the ion exchange process to occur properly. pH electrodes should always be stored in a moistened condition. When not in use, it’s best to store the electrode with the sensing surface fully immersed in either buffer 4.0 or buffer 7.0. Never store an electrode in distilled or deionized water as this will cause migration of the fill solution from the electrode. However, if an electrode should become dry, all is not lost. Many times, the electrode can simply be placed in some tap water for a half hour to recondition the glass.
So What Could Possibly Go Wrong?
Aging – pH electrodes are like batteries — they run down with time and use. As an electrode ages its glass changes resistance, with this change altering the electrode potential. For this reason, electrodes must be calibrated on a regular basis. Calibration in a pH buffer solution allows you to correct for this gradual change over time. Calibration of any pH equipment should always begin with buffer 7.0 as this is the “zero point.” A “dual” calibration using a second buffer solution of 4.0 or 10.0 provides for greater system accuracy. On the shelf, the electrode should last approximately a year if kept in a moistened condition.
Clogging – pH electrodes have junctions which allow the internal fill solution of the measuring electrode to leak out into the solution being measured. This junction can become clogged by particulates in the solution and can also facilitate poisoning by metal ions present in the solution. If a clogged junction is suspected it is best to soak the electrode in some warm tap water to dissolve the material and clear the junction.
Electrode Lifespan
Given all of the properties discussed above, you can see that pH electrodes have a finite lifespan. How long your pH electrode will last will depend entirely on how it is cared for and the solutions it is used to measure. The harsher the system, the shorter the lifespan. Typically, a gel-filled combination pH electrode will last six months to 1 year depending on the care and application. Remember – even if an electrode is not used it still ages!
Sensor Replacement
Electrode demise can usually be characterized by a sluggish response, erratic readings or a reading which will not change. When this occurs, an electrode can no longer be calibrated and must be replaced. For this reason it is always a good idea to have a back-up electrode on hand to avoid any system down time. Calibration is also an important part of electrode maintenance. This will assure that the electrode is behaving properly as it ages and that your system is operating correctly.
When it comes time to replace your pH electrode, Ryan Herco Flow Solutions can help you select the proper replacement unit for your specific application and OEM equipment. Just click or call us at 800-848-1141 for assistance with all your analytical instrumentation needs.